Catalytic reactions on metal surfaces are sensitive to the precise arrangement of atoms. Mechanical strains can influence the energetics of adsorption and desorption and, thus, the kinetics of catalytic reactions. We are studying the role of applied mechanical stress in modulating chemisorption energies at surface steps, a common location for many catalytic reactions. We have demonstrated that the chemisorption energy versus strain on steps is controlled by an intrinsically mechanical contribution that dominates any electronic effects. This is in contrast to the behavior on flat surfaces, where the electronic effect dominates. The mechanical contribution can have an entirely different trend (compression can induce stronger binding, whereas the electronic effect predicts that tension induces stronger bonding), and this differing trend can be used to increase catalytic activity. We have demonstrated this computationally for some important catalytic reactions.
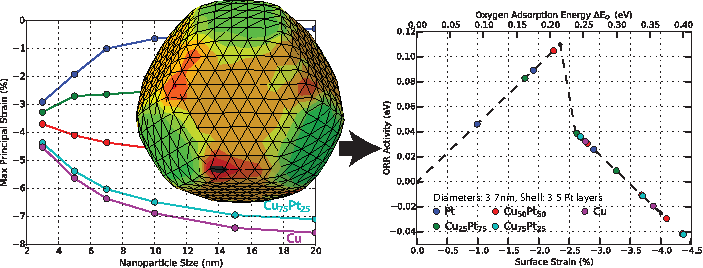
Surface strains in core–shell nanoparticles modify catalytic activity. We have created a continuum-based strategy which enables accurate surface-strain-based screening and design of core–shell systems using minimal input as a means to enhance catalytic activity. The approach is validated for Pt shells on Cu(x)Pt(1–x) cores and used to interpret experimental results on the oxygen reduction reaction in the same system. The analysis shows that precise control of particle sizes and shell thicknesses is required to achieve peak activity, rationalizing the limited increases in activity observed in experiments. The method is also applied to core–shell nanorods to demonstrate its wide applicability. The source code for this tool is available here: nanoparticle_surface_effects_v1.0.7z (435 mb).
Key publications:
- M. Francis and W. A. Curtin, New Trends in Catalytic Activity under Stress at Surface Steps, to appear in Nature Communications (2015).
- P. Moseley and W. A. Curtin, “Computational Design of Strain in Core-Shell Nanoparticles for Optimizing Catalytic Activity”, Nano Letters (2015).
- X. Zhang, G. Lu, and W. A. Curtin, “Multiscale Quantum/Atomistic Coupling Using Constrained Density Functional Theory”, Phys. Rev. B (2013). Some results in the published paper are not accurate. However, subsequent developments, in which the “penalty parameter” λ that enforces the constraint is extrapolated to infinity, yield results comparable to those quoted in the paper.